
In alignment with the federal Cannabis Act and Regulations, the OCS Client Services Team collects information regarding product quality and complaints. We use this information for quality assurance and customer care, to help with the investigation and resolution of complaints, and for fraud prevention purposes. Our suppliers are legally responsible for assuring the quality of their products and investigating all product complaints.

- All cannabis sold by OCS is procured only from federally licensed and regulated producers who follow strict guidelines. All cannabis products are tested in third party laboratories to ensure they are free from pesticides, micro-organisms and other items that could be harmful to a person’s health
- OCS reviews all product documentation from the laboratories for compliance with Health Canada regulations
- OCS has established a robust Quality Assurance team
- The Quality Assurance team provides an additional check on all cannabis products entering the Ontario market to ensure that it is compliant with all relevant regulations. This relieves the burden on retailers to quality assure products, and provides confidence to consumers that products are safe, reliable, and tested
We all play a role in delivering controlled, legal cannabis to consumers to avoid undue risk to the public. The OCS oversees the safe distribution of legal cannabis for the province of Ontario by reviewing the following:
Label & Package: Visual examination to confirm format and placement of mandated elements and provide feedback on non-conformances identified and possible resolution
Certificate of Analysis: Verify product release test results for conformance to specifications and approve and facilitate product shipments/purchase orders

OCS Vape Vetting Process
The OCS has put in place a strict vetting process to ensure our vape products are held to extremely high standards for quality and safety. The OCS Merchandising and Quality Assurance teams have worked with all licensed producers to validate hardware, production, finished product and packaging of vapes and vaping products to ensure all standards are met and consistency is upheld. Products have been evaluated on source flower, distillation method, hardware, terpene source and complexity, as well as overall product potency.
HARDWARE CERTIFICATION
Ensures electrical/mechanical integrity of the device during usage and transportation. These include, but are not limited to: UL8139, UL1642, CAN/CSA-E62133,UN/DOT38.3.
HARDWARE COMPONENTS
Ensures screening of potential contaminants. These include, but are not limited to: Microbials & Toxins, Heavy Metals & Plasticizers migration.
VAPE FORMULATION
Ensures screening of potential known harmful compounds/by-products. These include, but are not limited to: Pesticides, Microbials, Heavy Metals, Residual Solvents, Diacetyl, Diacetyl Derivatives, Formaldehydes, Acetaldehydes, Acrolein.
Submitting a Product Quality Assurance Claim to the OCS
OCS works with Licensed Producers on investigating all product quality complaints submitted by Licensed Retailers.
What is a Quality Assurance Claim?
1. Any alleged deficiency related to the identity, quality, durability, reliability, safety, effectiveness, labeling, packaging, or performance of a product after its release for distribution; or
2. Any product that does not meet the product specification or function in the manner intended.

RETAILER IDENTIFIES A PROBLEM WITH A CANNABIS PRODUCT
a. Product Quality Complaint;
b. Labeling/Packaging Complaint; or
c. Concealed Shortage/Damage
Please note: all complaints regarding accessories should be followed up directly with the accessory supplier. For more information or to obtain accessory supplier contact info, please contact the OCS Client Services Team at 1-877-627-1627.

RETAILER SUBMITS A PRODUCT QUALITY ASSURANCE CLAIM
The OCS Client Services Team collects information about product quality complaints in accordance with the Cannabis Act and Cannabis Regulations. We use this information for quality assurance and customer care to help with the investigation and resolution of complaints and for fraud prevention purposes. Our Licensed Producers (LPs) are legally responsible for assuring the quality of their products and investigating all Product complaints.
This step-by-step training video will help Retailers navigate the self-serve online claims process:
For more detailed information and FAQs about the online claims process and product quality assurance claims, please refer to the Submitting Product Claims and Claims Dashboard section of the Portal Guide.

OCS RECEIVES THE CLAIM AND WILL:
- File a formal Quality Assurance investigation with the Licensed Producer;
- Communicate the incident reference number to the Retailer; and
- Outline the next steps within the process to both Retailer and Producer

OCS SUBMITS A FORMAL INVESTIGATION WITH THE LICENSED PRODUCER
Licensed Producers will receive all complaints via the OCS Quality Assurance Team and are required to trend and monitor consumer feedback. All complaints will require an investigation form to be completed by the Licensed Producer in order to be closed.
The Licensed Producer must conduct an initial risk assessment as soon as reasonably practicable but, in any event, no later than 72 hours after receiving any customer complaint to determine if the product should continue to be available for sale until further investigation can be completed.
If a Product line or batch/lot number is deemed to be a Non-Conforming Product as a result of any quality investigation, the Licensed Producer must notify OCS Quality Assurance immediately so that appropriate control actions can be taken. If there is remediate action required to resolve the customer’s order, (including returns, refunds, investigation details to share, etc.), this information should be communicated to the OCS Quality Assurance Team. The Licensed Producer must adhere to all requirements for investigating and reporting adverse effects or safety incidents to Health Canada and other applicable regulatory bodies.
Please note: OCS also follows up with Licensed Producers regarding all filed Quality Assurance investigations that have not received a response in 30 days

LICENSED PRODUCER WILL RESPOND ONE OF THE FOLLOWING:
A. Credit Denied – and provide details of the investigation together with an explanation for the credit denial.
OR
B. Credit Approved – and OCS will provide resolution to the retailer regarding approval notice and credit note invoice. The credit will be applied on the account in a timely manner and used towards the following week’s order.
For product category information, refer to the following table.
Please Note: All Product Quality Assurance Claims with a visual defect must include a photo. This table is not exhaustive of all product issues.
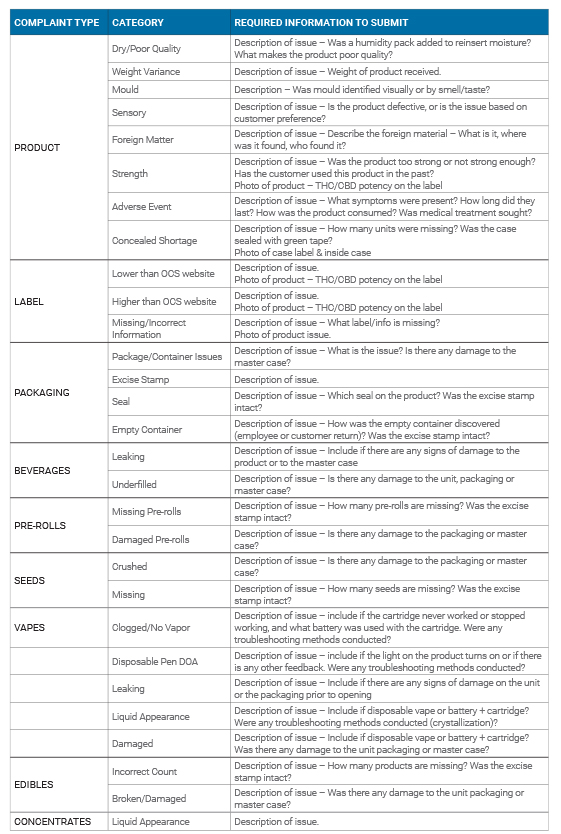
Please connect with OCS Client Services if a product is not listed. For more information regarding how to submit a Product Quality Complaint to the OCS please visit our Contact page or by calling 1-877-627-1627.

